Service
Bringing It All Together
This is the default dialog which is useful for displaying information. The dialog window can be moved, resized and closed with the 'x' icon.
Clinical Trials
- Data Management
- Biostatistics
- PK/PD
- eTMF Manager
- Medical Coding
- Drug Safety
- Quality Assurance
Data
- Data Analysis
- Data Pooling/Mining
- SDTM Conversion
- Data Mapping for CDISC/CDASH Standards
Systems Validation
- Clinical Systems validation techniques in compliance with 21CFR11 including Validation Master Plan (VMP) creation and IQ/OQ/PQ test script creation, review, and execution.
- System Audits
FEATURED PRODUCTS
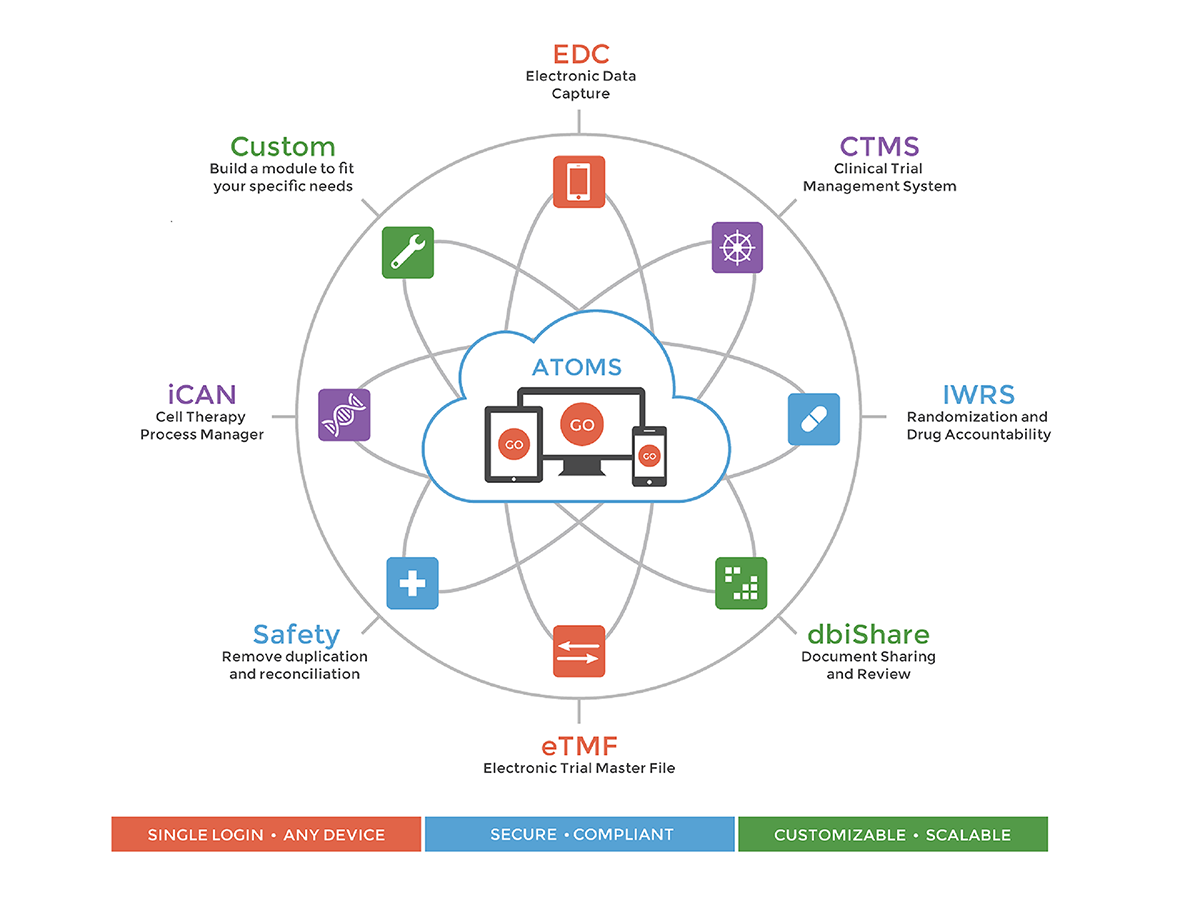
ATOMS
Fully integrated SDTM compliant and 21 CFR Part 11 compliant proprietary system which includes: Electronic Data Capture, CTMS, dbiShare, Safety Database, IWRS, Clinical Trial Material Management, Financial Tracker, and eTMF
About DBI
-
Database Integrations, Inc was founded in 2006 and is headquartered in Alpharetta, Georgia. We have an experienced staff with over 15 years of experience in Clinical Project Management, Data Management, Systems Validation, and Information Technology. We have extensive knowledge of regulatory requirements and have Experience in Phase I - IV clinical studies.
-
-
To change humanity by partnering in the discovery of life changing therapies

-
We are the industry leader that empowers confidence in all of our clients. We deliver exceptional service and solutions. We are the best investment our partners make.
-
-
Respect
We respect one another, recognizing our success depends on the commitment, capabilities, and diversity of our employees and partners
-
Teamwork
Our constant and never ending improvement attitude delivers top notch performance. We encourage collaboration, celebrate successes, and build and nurture long standing relationships.
-
Integrity
We are ethical and trustworthy in our relationships with partners and colleagues. We own and are accountable for results, success, and failures.
-
Innovation
We anticipate change and respond with creative solutions. We are agile and responsive to the changing needs of our partners and embrace every learning opportunity.
-
Excellence
We deliver quality service with every interaction and maximize each opportunity to provide the best possible customer experience. We strive to deliver the unexpected and know that details matter….it’s worth getting it right.
-
Giving Back
No one reaches their goals or becomes successful in a vacuum. We all depend on each other, our communities, and our country to help us grow, achieve and prosper. We understand that with success comes the responsibility to give back. We feel a responsibility to invest in the communities, institutions and people that have touched our lives.
Founders

Steve Littlefield, MBA
Co-founder
CEO/Creative Director
click for bio >
Steve Littlefield
Steve Littlefield is the co-founder of DBI and artist behind the development of the ATOMS platform. Steve has over 20 years of extensive experience in the information technology and information systems fields. For most of Steve’s career, he designed systems for various companies that could compile data sets from various groups and merge them to show combined output. During his more than 12 years within the pharmaceutical industries Clinical Systems and Data Management departments, he has also performed many nontraditional tasks such as system quality assurance audits, disparate dataset reconciliations, and regulatory eCDT submission preparation. As the Creative Director for DBI, Steve is responsible for leading the team in the creation and continual innovation of ATOMS as well as the leadership for continuing to build the business culture of impeccable customer service and delivering the unexpected. Steve has a Bachelor’s of Science Degree in Computer Engineering from Southern Polytechnic State University and an MBA from the University of Florida.

Jackie Littlefield, MSW, MBA
Co-founder
Chief Operating Officer
click for bio >
Jackie Littlefield
Jackie Littlefield is the co-founder of DBI and has over 15 years research experience in global drug development in pharmaceutical, biotech, medical device, contract research organization, and research sites. Her career has included roles for study coordinating, monitoring, clinical team lead, global project management, line management, and systems development. She is instrumental in ensuring clinical considerations are made with the evolution and innovation of the ATOMS platform. She also strategically leads DBI’s financial and operational departments and the delivery of high quality services in support of DBIs partnerships. Along with the other management team, Jackie is accountable for ensuring the quality, vibrancy and strategic alignment of DBI’s finances and administration with the mission, vision, and values of the company. She also plays a key role in the marketing and business development efforts. Jackie has a BSW from the University of West Florida, an MSW from Florida State University, and an MBA from the University of Florida.
PARTNERSHIPS
"Partnerships create new possibilities & opportunities which wouldn't otherwise exist"

DBI and Prima BioMed collaborate to commercialize iCAN

About SPRI
In 1972 two psychiatrist began SPRI as a clinical research site in Brooklyn, NY to bring research into the community. Forty years later the SPRI team continues that same patient centric focus to bring together science, technology, and patients to promote health on a global scale. As a clinical CRO with locations in underserved areas such as Georgia, Mexico, Russia, Turkey and Ukraine the team is able to ensure quality data and on time enrollment. Leveraging the team's experience and expertise in generics, CNS, infectious disease and oncology SPRI works with each client to develop flexible and scalable solutions that fit their needs.
Contact
Leave a Message
Contact Us
Database Integrations, Inc.6770 Jamestown Drive Alpharetta, GA 30005
Phone: (678) 829-1354
partners@dbintegrations.com

FOLLOW US